9701 MCQ Solution 72
Question 72: [Physical > Chemical Bonding]
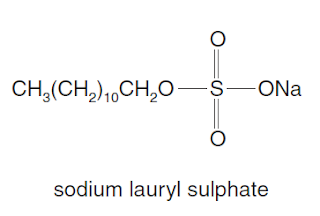
Long-chain alkanes are converted on an industrial scale into alkylsulphates for use as detergents,
e.g. sodium lauryl sulphate.
What deductions about the properties of this substance can be made from this structure?
1 Part of the structure is polar and is water–attracting.
2 The alkyl chain is soluble in oil droplets.
3 All the C-C-C bond angles are tetrahedral.
What deductions about the properties of this substance can be made from this structure?
1 Part of the structure is polar and is water–attracting.
2 The alkyl chain is soluble in oil droplets.
3 All the C-C-C bond angles are tetrahedral.
Reference: Past Exam Paper -June 2003 Paper 1 Q32
Solution 72:
Answer: A.
Option 1 is true that sodium lauryl suplhate is polar because it is made up of more than one type of atom and it does not have a symmetrical structure therefore has permanent dipoles.
Option 3 is also true because each C has four other single bonds which creates a tetrahedral structure.
Option 3 is also true because each C has four other single bonds which creates a tetrahedral structure.
.png)







0 comments :
Include ONLY the reference.
Comments will only be published after moderation