9701 MCQ Solution 65
Question 65: [Organic > Carbonyl Compounds]
Which reaction is not
an electrophilic addition?
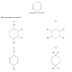
A CH2=CH2 + HI à CH3CH2I
B CH3CH=CH2 + Br2 à CH3CHBrCH2Br
C CH3CH=CH2 + H2O à CH3CH(OH)CH3
D CH3CHO + HCN à
CH3CH(OH)CN
Note: in option C
there is a catalyst - conc H2SO4
Reference: Past Exam Paper –June 2003 Paper 1 Q25
Solution 65:
Answer: D.
In organic reactions electrophilic addition occurs wherever
a double bond is broken. In option A, a double bond is replaced with H and I
atoms. In option B, the double bond is replaced with bromine atoms. In option
C, the double bonds are replaced with an H atom and an OH group. In option D,
there is no double bond and no electrophilic addition, since the reaction
between aldehyde and HCN is nucleophilic addition. Thus option D is the correct
answer.
.png)







0 comments :
Include ONLY the reference.
Comments will only be published after moderation