9701 MCQ Solution 26
Question 26:
[Organic > Hydrocarbons]:
Oxidation of an alkene Y
gives a diol; further oxidation gives a diketone.
What could be Y?
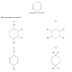
A CH3CH=C(CH3)2
B (CH3)2CHCH=CH2
C C6H5CH=CHC6H5
D (C6H5)2C=CHCH3
Reference: Past Exam Paper – November 2002 Paper 1 Q26
Solution 26:
Answer: C.
To form a diol the alkene would require that the unsaturated carbons each
have at least 1 hydrogen atom bonded to it.
Option A and D do not provide this
hence are eliminated.
In option B the compound has 2 hydrogen atoms bonded to 1
of the unsaturated carbons this will cause it to become partially a primary
alcohol and further oxidation will lead to fusion of a ketone from one side and
a carboxylic acid on the other not a diketone. Hence option B is also
incorrect.
Option C provides a secondary alcohol on both sides hence will
produce a diketone, making it the correct option.
.png)







0 comments :
Include ONLY the reference.
Comments will only be published after moderation