9701 MCQ Solution 6
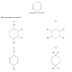
Which is the most likely shape of a molecule of hydrazine, N2H4?
Reference: Past Exam Paper – November 2002 Paper 1 Q6
Solution 6:
Answer: D.
The nitrogen atoms in hydrazine have 3 bonds. Each nitrogen
atom has 5 electrons in its outer shell. If 3 of these electrons are used to
form bonds, 1 with the other nitrogen atom, 1 with 1 hydrogen atom, 1 with
another hydrogen atom, it will leave 2 electrons in the nitrogen atom’s outer
shell. These 2 electrons will form a lone pair of electrons. Thus surround
either nitrogen atom are 3 bonded pair of electrons and 1 lone pair of
electrons. Thus the bond angle in either of the nitrogen atoms must 107, due to
the repulsion that this combination of lone and bonded pairs of electrons
causes.
.png)








0 comments :
Include ONLY the reference.
Comments will only be published after moderation