9701 MCQ Solution 4
Question 4: [Physical
> Bonding]:
The African weaver ant defends its territory by spraying an
intruder with a mixture of compounds. The ease by which these compounds are
detected by other ants depends upon the volatility, which decreases as the
strength of the intermolecular forces in the compound increases.
Which compound would be the most volatile?
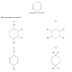
A CH3CH2CH2CH2CH2CH3
B CH3CH2CH2CH2CH2CHO
C CH3CH2CH2CH2CH2NH2
D CH3CH2CH2CH2CH2OH
Reference: Past Exam Paper – November 2002 Paper 1 Q4
Solution 4:
Answer: A.
The compound in answer A is a long chain hydrocarbon and the
only noticeable intermolecular forces are Van der Waals forces. The compound in
B has a C=O bond present which, though slight due to the large size of the
molecule, can cause a permanent dipole – dipole attraction to be setup. Thus the
compound in B is less volatile than the compound in A. Both compounds in C and
D have a hydrogen atom bonded to a nitrogen and oxygen atom, respectively. This
allows for the formation of hydrogen bonds between molecules of the compound
due to the difference in electro negativities between hydrogen and the other
atom. Hydrogen bonds are the strongest intermolecular force thus both these
options are eliminated. Hence correct answer is A.
.png)







0 comments :
Include ONLY the reference.
Comments will only be published after moderation