9701 MCQ Solution 5
Question 5: [Physical
> Bonding]:
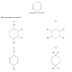
Which of the following molecules has no permanent dipole?
A CCl2F2
B CHCl3
C C2Cl4
D C2H5Cl
Reference: Past Exam Paper – November 2002 Paper 1 Q5
Solution 5:
Answer: C
The compound in A is a halogenoalkene that can exhibit
cis-trans isomerism. Due to this it may or may not have a permanent dipole thus
option A is eliminated. The compound in B has 1 carbon atom bonded to 3
chlorine atoms and 1 hydrogen atom. The difference in electronegativity between
the hydrogen and carbon atom will not match the difference in electronegativity
between the opposite chlorine and carbon atom. Due to this mismatch the
molecule will overall exhibit a partial charge and thus a permanent dipole,
eliminating option B as a correct answer. The compound in C does not exhibit
cis-trans isomerism due to the fact that the unsaturated carbon atoms are both
bonded to 4 identical chlorine atoms. All differences in electronegativities
cancel out thus no overall permanent dipole. This means that C is the correct
answer. For arguments sake, the compound in D has 5 hydrogen atoms bonded to a
carbon atoms and 1 chlorine atom bonded to a carbon atom, similar to the
scenario in compound B, this will cause a permanent dipole thus eliminates D as
the correct answer.
.png)







0 comments :
Include ONLY the reference.
Comments will only be published after moderation