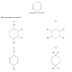
Question 80: [Organic > Carboxylic Acids and Derivatives]
Mevalonic acid is an intermediate in the biosynthesis of
cholesterol, and is shown below.
Which properties does mevalonic acid have?
1 It has only 1 chiral carbon atom.
2 It can be esterified both by ethanoic acid and by ethanol,
in the presence of H+ ions.
3 It contains both primary and secondary alcohol groups.
Reference: Past Exam Paper –June 2003 Paper 1 Q40
Solution 80:
Answer: B.
Fact 1 is correct since the central carbon atom is bonded to
4 different groups, whereas the remaining carbon atoms have bonds to 2 or more
H atoms, or have a double bond to an oxygen atom. Fact 2 is also correct, since
mevalonic acid has a carboxylic acid group on the left-hand side and an alcohol
group on the right-hand side. This allows esterification by both ethanolic acid
and ethanol. Fact 3 is incorrect since mevalonic acid has a primary alcohol (2
or more H atoms) group on the right-hand side, and a tertiary alcohol (No H
atoms) in the centre. Thus option B is the correct answer.
Question 79: [Organic > Hydroxyl Compounds]
When the apparatus below was used with compound Z, a brick-red precipitate formed in
the right-hand tube.
Which compound could be Z?
1 CH3CH(OH)CH3
2 CH3CH2CH2OH
3 CH3OH
Reference: Past Exam Paper –June 2003 Paper 1 Q39
Solution 119:
Answer: C.
1, 2 and 3 are all alcohols. When an alcohol is presented to
heated acidified potassium dichromate they will oxidise. In 1, the alcohol has
1 H atom bonded to the alcohol-bearing C atom and is secondary which will form
a ketone. In 2 and 3 the alcohols have 2 or more H atoms bonded to the
alcohol-bearing C atom and are primary which will form aldehydes. Aldehydes
turn into a brick red precipitate when presented to Fehling’s reagent. Thus 2
and 3 are the only possible compounds that Z
can be. Thus the correct answer is option C
Question 70: [Organic > Carbonyl Compounds]
The product of the reaction between propanone and hydrogen
cyanide is hydrolysed under acidic conditions.
What is the formula of the final product?
A CH3CH(OH)CO2H
B CH3CH2CH2CO2H
C (CH3)2CHCONH2
D (CH3)2C(OH)CO2H
Reference: Past Exam Paper –June 2003 Paper 1 Q30
Solution 70:
Answer: D.
First observe the formula for propanone: (CH3)2C=O.
The reactants undergo nucleophilic addition. The CN from HCN bonds with the
central carbon atom, breaking the double bond with oxygen into a single bond.
The oxygen has a polar charge attracting the remaining H atom from HCN, to form
an alcohol group. Under acidic conditions the CN group is oxidised into a
carboxylic acid. This is best shown by the product in option D.
Question 69: [Organic > Hydroxyl Compounds]
In a preparation of ethene, ethanol is added a drop at a
time to a heated reagent Y. To
purify the ethane it is bubbled through a solution Z and then collected.
What could reagent reagent Y and solution Z be?
|
|
reagent Y
|
solution Z
|
|
A
|
acidified K2Cr2O7
|
dilute NaOH
|
|
B
|
concentrated H2SO4
|
dilute H2SO4
|
|
C
|
concentrated H2SO4
|
dilute NaOH
|
|
D
|
ethanolic NaOH
|
concentrated H2SO4
|
Reference: Past Exam Paper –June 2003 Paper 1 Q29
Solution 69:
Answer: C.
Alcohol undergoes dehydration to form an alkene.
Concentrated H2SO4 is among the possible conditions for
this reaction. This eliminates options A and D as possible answers. After
dehydration occurs there may be remaining H2SO4 mixed
with the alkene. This remainder must be neutralised. It is acidic and in option
B, the addition of more H2SO4 will contaminate the alkene
more, thus is an incorrect answer. A basic solution will neutralise the acid,
such as the dilute NaOH in option C, which is the correct answer.
Question 68: [Organic > Carbonyl Compounds]
In its reaction with sodium, 1 mol of a compound X gives 1 mol of H2(g).
Which compound might X
be?
A CH3CH2CH2CH2OH
B (CH3)3COH
C CH3CH2CH2CO2H
D CH3CH(OH)CO2H
Reference: Past Exam Paper –June 2003 Paper 1 Q28
Solution 68:
Answer: D.
1 alcohol group reacts with 1 Na atom to produce 1 hydrogen
atom. The same occurs for 1 carboxylic acid group. 2 form 1 mole of H2
the compound would need to produce 2 H atoms. This can only be done if the
compound has 2 alcohol groups, or 2 carboxylic acid groups, or 1 of each. In
option A, there is only 1 alcohol group. In option B, there is also only 1
alcohol group. In option C, there is 1 carboxylic acid group. In option D,
there is 1 carboxylic acid group and 1 alcohol group. Thus option D is the
correct answer.
Question 67: [Organic > Halogen Derivatives]
Chlorofluoroalkanes, commonly known as CFCs, undergo
homolytic fission by ultraviolet irradiation in the stratosphere.
Which radical could result from the irradiation of CHFClCF2Cl?
A CHFClĊFCl
B ĊHClCF2Cl
C ĊHFCF2Cl
D ĊFClCF2Cl
Reference: Past Exam Paper –June 2003 Paper 1 Q27
Solution 67:
Answer: C.
In the given CFC above, there are C-H bonds, C-F bonds, C-Cl
bonds and a C-C bond. In all the options the C-C bond remains intact. The order
of bond strength of the remaining 3 bonds is: C-H>C-F>C-Cl. Thus a C-Cl
bond will be broken since it requires the least energy. This only occurs in
option C. In option A, the CFC loses a F atom, suggesting that a C-F bond is
broken. In option B, The CFC loses a different F atom, yet still suggesting
that a C-F bond is broken. In option D, the CFC loses a H atom, suggesting that
a C-H bond is broken. According to the order of bond strengths these options
are incorrect. Thus option C is the correct answer.
Question 66: [Organic > Halogen Derivatives]
The reaction scheme outlines the production of one of the
monomers of nylon 66 from compound X.
Which compound could be X?
A BrCH2CH2CH2CH2Br
B CH2=CHCH=CH2
C HOCH2CH2CH2CH2OH
D HO2CCH2CH2CH2CH2CO2H
Reference: Past Exam Paper –June 2003 Paper 1 Q26
Solution 66:
Answer: A.
In option A, the halogenoalkane has Br atoms which are
replaced with CN groups by nucleophilic substitution. In option B, the alkene
does not react with KCN. In option C, the alcohol does not react with KCN. In
option D, the carboxylic acid does not react with KCN. Thus option A is the
correct answer.
Question 65: [Organic > Carbonyl Compounds]
Which reaction is not
an electrophilic addition?
A CH2=CH2 + HI à CH3CH2I
B CH3CH=CH2 + Br2 à CH3CHBrCH2Br
C CH3CH=CH2 + H2O à CH3CH(OH)CH3
D CH3CHO + HCN à
CH3CH(OH)CN
Note: in option C
there is a catalyst - conc H2SO4
Reference: Past Exam Paper –June 2003 Paper 1 Q25
Solution 65:
Answer: D.
In organic reactions electrophilic addition occurs wherever
a double bond is broken. In option A, a double bond is replaced with H and I
atoms. In option B, the double bond is replaced with bromine atoms. In option
C, the double bonds are replaced with an H atom and an OH group. In option D,
there is no double bond and no electrophilic addition, since the reaction
between aldehyde and HCN is nucleophilic addition. Thus option D is the correct
answer.
Question 64: [Organic > Halogen Derivatives]
Cyclohexa-1,4-diene is treated with a solution of bromine in
tetrachloromethane.
Reference: Past Exam Paper –June 2003 Paper 1 Q24
Solution 64:
Answer: B.
Double bond present on the right and left sides of
cyclohexa-1,4diene. They undergo electrophilic addition due to presence of
bromine molecules. 4 carbon atoms on the sides are unsaturated, where the
bromine atoms will be added. This is depicted best by option B. In option A,
the 2 bromine atoms on the sides are correctly placed, however the bromine
atoms on the top and bottom are misplaced since the carbon atoms they are
bonded to were already saturated. In option D, all the bromine atoms are
misplaced for the same reason as the top and bottom bromine atoms in option A.
In option C, the double bonds never broke which is completely illogical as they
must break in presence of bromine solution. Thus options A, C and D are all
incorrect.
Question 103: [Organic > Hydrocarbons]
The complete combustion of alkanes to produce carbon dioxide
and water is an important exothermic reaction.
Which line on the graph shows the relationship between the
number of carbon atoms in the alkane and the number of moles of oxygen gas
needed for complete combustion of the alkane?
Reference: Past Exam Paper –June 2003 Paper 1 Q23
Solution 63:
Answer: B.
1 mole of oxygen gas has 2 oxygen atoms. In the combustion
of 1 carbon atom, 1 mole of CO2 is formed. This requires 1 carbon
atom and 2 oxygen atoms. Thus 2 carbon atoms requires 2 moles of oxygen gas
(which actually has 4 atoms of oxygen), to form 2 moles of CO2. Hence
the number of moles of oxygen gas and number of carbon atoms are directly
proportional, in a 1:1 ratio. This is only depicted by the line in option B.
Option A and C are curved thus do not show a constant proportionality, thus are
eliminated. Option D suggests the amount of oxygen gas required for the
combustion of any number of carbon atoms is the same, which is illogical, thus
is eliminated.
.png)